
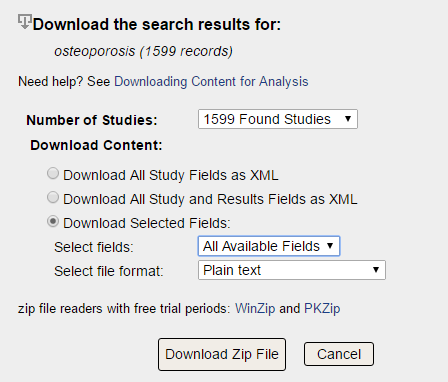
Ideally, complete and accurate key study information (eg, study design, recruitment information) is registered at the start of the study and updated throughout the research life cycle, and summary results are reported after the study is completed. Sponsors and investigators are responsible for submitting information about their studies to. 1 The goal of the trial reporting system is to provide a public mechanism for identifying and characterising all trials conducted to answer specific biomedical questions (that is, the so-called denominator for all such trials) and their summary findings (that is, the evidence base). The database, which is maintained by the National Library of Medicine at the National Institutes of Health (NIH), consists of a clinical study registry and results database-two principal components of the trial reporting system. This article discusses 10 key issues that researchers need to consider when using the database to conduct research.Ĭ is a web based resource providing access to summary information on publicly and privately supported clinical studies on a wide range of diseases and conditions. Conducting valid analyses requires an understanding of both the capabilities and limitations of the database (that is, intrinsic factors) as well as reporting policies and other factors external to the database that influence the types of studies in in a specified time.
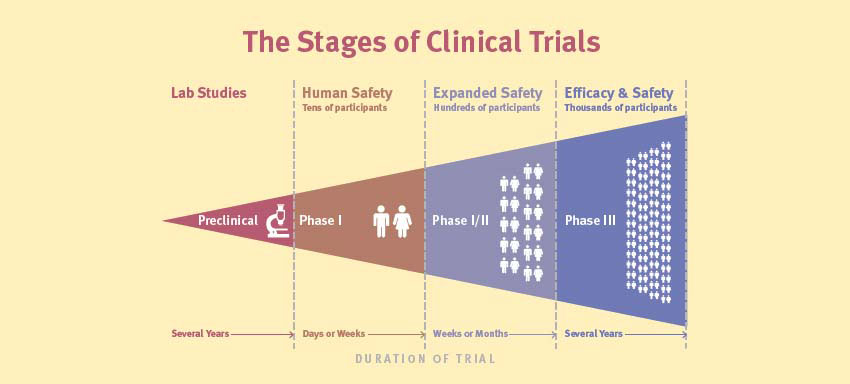
Researchers are increasingly using information from the database to assess research reporting practices, or to characterise the clinical research enterprise. , a repository of information about clinical studies and their results, together with specialised search tools, provides a unique window into the clinical research enterprise, which includes all initiated, ongoing, and completed or terminated clinical studies.


 0 kommentar(er)
0 kommentar(er)
